
- Mechanism of actoion for ifactor bone graft update#
- Mechanism of actoion for ifactor bone graft trial#
Two primary outcome measures were assessed: cost and utility. We analyzed various real-world scenarios, including inpatient and outpatient surgical settings as well as private versus public insurances. Patients randomly received either autograft (N = 154) or i-Factor (N = 165).
Mechanism of actoion for ifactor bone graft trial#
Methods: The patient cohort was extracted from a prospective, multicenter randomized control trial (RCT) from twenty-two North American centers. To evaluate the cost-effectiveness of i-FACTOR compared to autograft for patients undergoing ACDF surgery. Cost-effectiveness of these innovations remains an often-overlooked aspect of this effort.

Objective: The efficacy and safety of traditional anterior cervical discectomy and fusion (ACDF) surgery has improved with the introduction of new implants and compounds. Study Design: We conducted decision analytical modeling using a Markov model to determine the ICER of i-factor compared to autograft in ACDF surgery. Neuronomics LLC, 7320 Woodlake Ave, Suite 215, West Hills, CA, 91307, USA P-15L Bone Graft is an Investigational Product limited by Federal (USA) Law to Investigational Use.Bart Thaci, 1 Randy Yee, 2 Kee Kim, 1 Amir Vokshoor, 2– 4 J Patrick Johnson, 5 Jared Ament 2– 5ġUniversity of California, Davis, Sacramento, CA, USA 2Neuronomics LLC, Los Angeles, CA, USA 3Neurosurgery & Spine Group, Los Angeles, CA, USA 4Institute of Neuro Innovation, Santa Monica, CA, USA 5Cedars Sinai Medical Center, Los Angeles, CA, USA This novel mechanism of action is designed to support safer and more predictable bone formation compared to commercially available bone growth factors. i-FACTOR Peptide Enhanced Bone Graft is the only biologic bone graft in orthopedics that incorporates a small peptide as an attachment factor to stimulate the natural bone healing process. "Increasingly surgeons and hospitals are recognizing the advantages of the i‑FACTOR Peptide Enhanced Bone Graft's biomimetic small peptide technology in helping to improve safety and outcomes, and we hope this ISASS policy statement builds awareness among healthcare institutions and payors of the proven benefits of our technology and the importance of making it a covered treatment option for patients."Ĭerapedics is an orthobiologics company developing and commercializing its proprietary synthetic small peptide (P-15) technology platform. suffer from degenerative disk disease that leads to pain and nerve irritation and often requires surgery," added Jeffrey Marx, Ph.D., president and chief operating officer of Cerapedics. "i‑FACTOR Peptide Enhanced Bone Graft has a wealth of clinical evidence supporting its use as well as the potential to offer an economical alternative that aligns with the values of healthcare systems." of Neurosurgery, University of Illinois Carle School of Medicine. "We are continually hearing about the development of new technologies to support bone regrowth and repair, but little about the supporting clinical evidence," said Paul M.
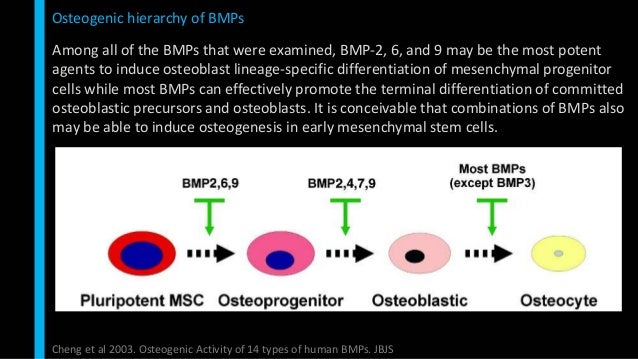
Cerapedics recently initiated a second investigational device exemption clinical trial to evaluate the safety and efficacy of the P-15 technology compared to autograft in transforaminal lumbar interbody fusion surgery. Prior research indicates that i‑FACTOR Peptide Enhanced Bone Graft is superior to the historical "gold standard" autograft in overall clinical success. I‑FACTOR Peptide Enhanced Bone Graft has a novel mechanism of action (attract, attach, and activate) that induces osteoblast cell proliferation and differentiation to accelerate new bone formation in patients with degenerative disk disease. "The ISASS statement notes that i‑FACTOR Peptide Enhanced Bone Graft is based on a well-established mechanism of action and carries extensive clinical data including level 1 human data that strongly supports its safety and efficacy."
Mechanism of actoion for ifactor bone graft update#
"We are very pleased that ISASS has issued this important policy update that differentiates our proprietary biomimetic small peptide (P-15) technology from other bone grafting products that have little or no clinical evidence," said Glen Kashuba, CEO of Cerapedics. Food and Drug Administration (FDA) through the Premarket Approval (PMA) process and supported by level 1 clinical data. Cerapedics, a privately-held orthobiologics company, said the International Society for the Advancement of Spine Surgery (ISASS) has issued a new bone grafting policy with recommendations on usage and payor coverage criterion that features i‑FACTOR Peptide Enhanced Bone Graft as one of only two drug-device combination products approved by the U.S.


 0 kommentar(er)
0 kommentar(er)
